Parkinson's Disease
To induce Parkinsonism in rodents for the identification and development of new therapies, we utilize the rotenone rat model. Our experience with protocols that offer dose-related, graded dopaminergic depletions and the ability to assess endpoints at the behavioural, biochemical and anatomical level, provides opportunities to study the effects of an NCE against these endpoints.
In the rat, the systemic injection of rotenone results in a variety of behavioural, biochemical and histopathological changes which bear parallels with changes in Parkinson’s Disease. Together these measures provide multiple endpoints to assess the effect of an NCE in this model.
Validation Data: Effect of Rotenone dose on indices of neurological function
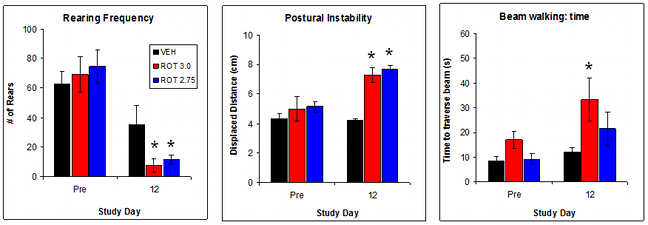
A 10-12 daily regimen of rotenone (2.75 – 3 mg/kg IP) produces significant effects on tasks of neurological function The most robust changes are measured using rearing in open field, postural instability and beam walking. Tremor, hypolocomotion, catalepsy are inconsistently affected by rotenone treatment.